Fungal Assemblages on Indoor Surfaces with Visible Mold Growth in Homes after the 2016 Flood Disaster in Thailand
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. DNA Extraction
2.3. DNA Sequencing
2.4. Sequence Procsesing and Analyses
3. Results
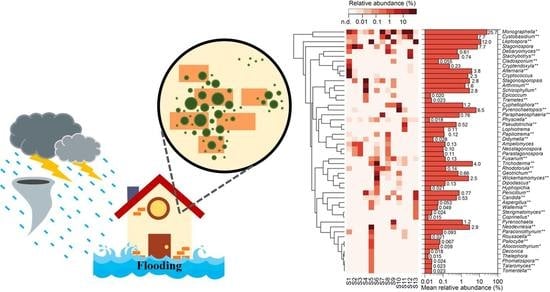
3.1. Fungal Diversity
3.2. Fungal Assemblages
3.3. Predominant Fungal Genera
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meteorological Department. The Climate of Thailand. Available online: https://www.tmd.go.th/en/archive/thailand_climate.pdf (accessed on 20 July 2020).
- Becher, R.; Hongslo, J.K. Biological pollution of indoor air. Tidsskr Nor Laegeforen 1994, 114, 2722–2724. [Google Scholar] [PubMed]
- Rao, C.Y.; Riggs, M.A.; Chew, G.L.; Muilenberg, M.L.; Thorne, P.S.; Van Sickle, D.; Dunn, K.H.; Brown, C. Characterization of airborne molds, endotoxins, and glucans in homes in New Orleans after Hurricanes Katrina and Rita. Appl. Environ. Microbiol. 2007, 73, 1630–1634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, N.Y.; Chen, P.Y.; Chang, H.W.; Su, H.J. Changes in profiles of airborne fungi in flooded homes in southern Taiwan after Typhoon Morakot. Sci. Total Environ. 2011, 409, 1677–1682. [Google Scholar] [CrossRef]
- Azuma, K.; Ikeda, K.; Kagi, N.; Yanagi, U.; Hasegawa, K.; Osawa, H. Effects of water-damaged homes after flooding: Health status of the residents and the environmental risk factors. Int. J. Environ. Health Res. 2014, 24, 158–175. [Google Scholar] [CrossRef] [PubMed]
- Emerson, J.B.; Keady, P.B.; Brewer, T.E.; Clements, N.; Morgan, E.E.; Awerbuch, J.; Miller, S.L.; Fierer, N. Impacts of flood damage on airborne bacteria and fungi in homes after the 2013 Colorado Front Range flood. Environ. Sci. Technol. 2015, 49, 2675–2684. [Google Scholar] [CrossRef] [PubMed]
- Beezhold, D.H.; Green, B.J.; Blachere, F.M.; Schmechel, D.; Weissman, D.N.; Velickoff, D.; Hogan, M.B.; Wilson, N.W. Prevalence of allergic sensitization to indoor fungi in West Virginia. Allergy Asthma Proc. 2008, 29, 29–34. [Google Scholar] [CrossRef]
- Rogers, C.A. Indoor fungal exposure. Immunol. Allergy Clin. N. Am. 2003, 23, 501–518. [Google Scholar] [CrossRef]
- Mi, Y.H.; Norback, D.; Tao, J.; Mi, Y.L.; Ferm, M. Current asthma and respiratory symptoms among pupils in Shanghai, China: Influence of building ventilation, nitrogen dioxide, ozone, and formaldehyde in classrooms. Indoor Air 2006, 16, 454–464. [Google Scholar] [CrossRef]
- Arundel, A.V.; Sterling, E.M.; Biggin, J.H.; Sterling, T.D. Indirect health effects of relative humidity in indoor environments. Environ. Health Perspect. 1986, 65, 351–361. [Google Scholar] [CrossRef] [Green Version]
- Baughman, A.V.; Arens, E.A. Indoor humidity and human health-Part I: Literature review of health effects of humidity-influenced indoor pollutants. ASHRAE Trans. 1996, 102, 193–211. [Google Scholar]
- Solomon, G.M.; Hjelmroos-Koski, M.; Rotkin-Ellman, M.; Hammond, S.K. Airborne mold and endotoxin concentrations in New Orleans, Louisiana, after flooding, October through November 2005. Environ. Health Perspect. 2006, 114, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Apisarnthanarak, A.; Khawcharoenporn, T.; Mundy, L.M. Black-water floods and hospital-based postflood mold investigations. Infect. Control Hosp. Epidemiol. 2012, 33, 1266–1268. [Google Scholar] [CrossRef] [PubMed]
- Khawcharoenporn, T.; Apisarnthanarak, A.; Thongphubeth, K.; Yuekyen, C.; Damnin, S.; Hayden, M.K.; Weinstein, R.A. Post-flood measurement of fungal bio-aerosol in a resource-limited hospital: Can the settle plate method be used? J. Hosp. Infect. 2013, 83, 150–152. [Google Scholar] [CrossRef] [PubMed]
- Luksamijarulkul, P.; Ratthanakhot, Y.; Vatanasomboon, P. Microbial counts and particulate matter levels in indoor air samples collected from a child home-care center in Bangkok, Thailand. J. Med. Assoc. Thai. 2012, 95 (Suppl. 6), S161-8. [Google Scholar]
- Siwarom, S.; Puranitee, P.; Plitponkarnpim, A.; Manuyakorn, W.; Sinitkul, R.; Arj-Ong Vallipakorn, S. Association of indoor air quality and preschool children’s respiratory symptoms. Asian Pac. J. Allergy Immunol. 2017, 35, 119–126. [Google Scholar] [CrossRef]
- Mendell, M.J.; Mirer, A.G.; Cheung, K.; Tong, M.; Douwes, J. Respiratory and allergic health effects of dampness, mold, and dampness-related agents: A review of the epidemiologic evidence. Environ. Health Perspect. 2011, 119, 748–756. [Google Scholar] [CrossRef]
- An, C.; Yamamoto, N. Fungal compositions and diversities on indoor surfaces with visible mold growths in residential buildings in the Seoul Capital Area of South Korea. Indoor Air 2016, 26, 714–723. [Google Scholar] [CrossRef]
- Hospodsky, D.; Yamamoto, N.; Peccia, J. Accuracy, precision, and method detection limits of quantitative PCR for airborne bacteria and fungi. Appl. Environ. Microbiol. 2010, 76, 7004–7012. [Google Scholar] [CrossRef] [Green Version]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef] [Green Version]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for Basidiomycetes: Application to the identification of mycorrhizae and rusts. Mol. Ecol. Notes 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsson, R.H.; Tedersoo, L.; Ryberg, M.; Kristiansson, E.; Hartmann, M.; Unterseher, M.; Porter, T.M.; Bengtsson-Palme, J.; Walker, D.M.; de Sousa, F.; et al. A comprehensive, automatically updated fungal ITS sequence dataset for reference-based chimera control in environmental sequencing efforts. Microbes Environ. 2015, 30, 145–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afgan, E.; Baker, D.; van den Beek, M.; Blankenberg, D.; Bouvier, D.; Čech, M.; Chilton, J.; Clements, D.; Coraor, N.; Eberhard, C.; et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2016 update. Nucleic Acids Res. 2016, 44, W3–W10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Dannemiller, K.; Reeves, D.; Bibby, K.; Yamamoto, N.; Peccia, J. Fungal high-throughput taxonomic identification tool for use with next-generation sequencing (FHiTINGS). J. Basic Microbiol. 2014, 54, 315–321. [Google Scholar] [CrossRef]
- Schoch, C.; Robbertse, B.; Robert, V.; Vu, T.; Cardinali, G.; Irinyi, L.; Meyer, W.; Nilsson, R.H.; Hughes, K.; Miller, A.N.; et al. Finding needles in haystacks: Linking scientific names, reference specimens and molecular data for Fungi. Database 2014, 1–21. [Google Scholar] [CrossRef]
- Bornehag, C.G.; Blomquist, G.; Gyntelberg, F.; Järvholm, B.; Malmberg, P.; Nordvall, L.; Nielsen, A.; Pershagen, G.; Sundell, J. Dampness in buildings and health. Nordic interdisciplinary review of the scientific evidence on associations between exposure to “dampness” in buildings and health effects (NORDDAMP). Indoor Air 2001, 11, 72–86. [Google Scholar] [CrossRef]
- DeLeon-Rodriguez, N.; Lathem, T.L.; Rodriguez, R.L.; Barazesh, J.M.; Anderson, B.E.; Beyersdorf, A.J.; Ziemba, L.D.; Bergin, M.; Nenes, A.; Konstantinidis, K.T. Microbiome of the upper troposphere: Species composition and prevalence, effects of tropical storms, and atmospheric implications. Proc. Natl. Acad. Sci. USA 2013, 110, 2575–2580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, W.; FitzGerald, G.J.; Clark, M.; Hou, X.Y. Health impacts of floods. Prehosp. Disaster Med. 2010, 25, 265–272. [Google Scholar] [CrossRef]
- IOM. Damp Indoor Spaces and Health; National Academy Press: Washington, DC, USA, 2005. [Google Scholar]
- Fouquier, J.; Schwartz, T.; Kelley, S.T. Rapid assemblage of diverse environmental fungal communities on public restroom floors. Indoor Air 2016, 26, 869–879. [Google Scholar] [CrossRef]
- Hegarty, B.; Haverinen-Shaughnessy, U.; Shaughnessy, R.J.; Peccia, J. Spatial gradients of fungal abundance and ecology throughout a damp building. Environ. Sci. Technol. Lett. 2019, 6, 329–333. [Google Scholar] [CrossRef]
- Fu, X.; Li, Y.; Yuan, Q.; Cai, G.-h.; Deng, Y.; Zhang, X.; Norbäck, D.; Sun, Y. Continental-scale microbiome study reveals different environmental characteristics determining microbial richness, composition, and quantity in hotel rooms. mSystems 2020, 5, e00119-20. [Google Scholar] [CrossRef]
- Hamilos, D.L. Allergic fungal rhinitis and rhinosinusitis. Proc. Am. Thorac. Soc. 2010, 7, 245–252. [Google Scholar] [CrossRef]
- Portnoy, J.M.; Kwak, K.; Dowling, P.; VanOsdol, T.; Barnes, C. Health effects of indoor fungi. Ann. Allergy Asthma Immunol. 2005, 94, 313–319. [Google Scholar] [CrossRef]
- Brown, G.D.; Denning, D.W.; Levitz, S.M. Tackling human fungal infections. Science 2012, 336, 647. [Google Scholar] [CrossRef] [Green Version]
- Badiee, P.; Hashemizadeh, Z. Opportunistic invasive fungal infections: Diagnosis & clinical management. Indian J. Med. Res. 2014, 139, 195–204. [Google Scholar]
- Caillaud, D.; Leynaert, B.; Keirsbulck, M.; Nadif, R.; mould_ANSES_working_group. Indoor mould exposure, asthma and rhinitis: Findings from systematic reviews and recent longitudinal studies. Eur. Respir. Rev. 2018, 27, 170137. [Google Scholar] [CrossRef] [Green Version]
- Hernberg, S.; Sripaiboonkij, P.; Quansah, R.; Jaakkola, J.J.; Jaakkola, M.S. Indoor molds and lung function in healthy adults. Respir. Med. 2014, 108, 677–684. [Google Scholar] [CrossRef] [Green Version]
- Hodgson, M.J.; Morey, P.; Leung, W.Y.; Morrow, L.; Miller, D.; Jarvis, B.B.; Robbins, H.; Halsey, J.F.; Storey, E. Building-associated pulmonary disease from exposure to Stachybotrys chartarum and Aspergillus versicolor. J. Occup. Environ. Med. 1998, 40, 241–249. [Google Scholar] [CrossRef]
- Johanning, E.; Biagini, R.; Hull, D.; Morey, P.; Jarvis, B.; Landsbergis, P. Health and immunology study following exposure to toxigenic fungi (Stachybotrys chartarum) in a water-damaged office environment. Int. Arch. Occup. Environ. Health 1996, 68, 207–218. [Google Scholar] [CrossRef]
- Simon-Nobbe, B.; Denk, U.; Poll, V.; Rid, R.; Breitenbach, M. The spectrum of fungal allergy. Int. Arch. Allergy Immunol. 2008, 145, 58–86. [Google Scholar] [CrossRef]
- Molnár-Gábor, E.; Dóczi, I.; Hatvani, L.; Vágvölgyi, C.; Kredics, L. Isolated sinusitis sphenoidalis caused by Trichoderma longibrachiatum in an immunocompetent patient with headache. J. Med. Microbiol. 2013, 62, 1249–1252. [Google Scholar] [CrossRef] [Green Version]
- Sandoval-Denis, M.; Sutton, D.A.; Cano-Lira, J.F.; Gene, J.; Fothergill, A.W.; Wiederhold, N.P.; Guarro, J. Phylogeny of the clinically relevant species of the emerging fungus Trichoderma and their antifungal susceptibilities. J. Clin. Microbiol. 2014, 52, 2112–2125. [Google Scholar] [CrossRef] [Green Version]
- Chowdhary, A.; Perfect, J.; de Hoog, G.S. Black molds and melanized yeasts pathogenic to humans. Cold Spring Harb. Perspect. Med. 2015, 5, a019570. [Google Scholar] [CrossRef] [Green Version]
- Revankar, S.G.; Sutton, D.A. Melanized fungi in human disease. Clin. Microbiol. Rev. 2010, 23, 884–928. [Google Scholar] [CrossRef] [Green Version]
- Edite Bezerra da Rocha, M.; Freire, F.d.C.O.; Erlan Feitosa Maia, F.; Izabel Florindo Guedes, M.; Rondina, D. Mycotoxins and their effects on human and animal health. Food Control 2014, 36, 159–165. [Google Scholar] [CrossRef]
- Hossain, M.A.; Ahmed, M.S.; Ghannoum, M.A. Attributes of Stachybotrys chartarum and its association with human disease. J. Allergy Clin. Immunol. 2004, 113, 200–208. [Google Scholar] [CrossRef]
- De Almeida, G.M.; Costa, S.F.; Melhem, M.; Motta, A.L.; Szeszs, M.W.; Miyashita, F.; Pierrotti, L.C.; Rossi, F.; Burattini, M.N. Rhodotorula spp. isolated from blood cultures: Clinical and microbiological aspects. Med. Mycol. 2008, 46, 547–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wirth, F.; Goldani, L.Z. Epidemiology of Rhodotorula: An emerging pathogen. Interdiscip. Perspect. Infect. Dis. 2012, 2012, 465717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaller, M.A.; Diekema, D.J.; Gibbs, D.L.; Newell, V.A.; Ellis, D.; Tullio, V.; Rodloff, A.; Fu, W.; Ling, T.A.; Global Antifungal Surveillance, G. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: A 10.5-year analysis of susceptibilities of Candida species to fluconazole and voriconazole as determined by CLSI standardized disk diffusion. J. Clin. Microbiol. 2010, 48, 1366–1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canton, E.; Peman, J.; Quindos, G.; Eraso, E.; Miranda-Zapico, I.; Alvarez, M.; Merino, P.; Campos-Herrero, I.; Marco, F.; de la Pedrosa, E.G.; et al. Prospective multicenter study of the epidemiology, molecular identification, and antifungal susceptibility of Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis isolated from patients with candidemia. Antimicrob. Agents Chemother. 2011, 55, 5590–5596. [Google Scholar] [CrossRef] [Green Version]
- De Hoog, S.; Mayser, P.; Haase, G.; Horré, R.; Horrevorts, A. A new species, Phialophora europaea, causing superficial infections in humans. Mycoses 2000, 43, 409–416. [Google Scholar] [CrossRef]
- Saunte, D.M.; Tarazooie, B.; Arendrup, M.C.; de Hoog, G.S. Black yeast-like fungi in skin and nail: It probably matters. Mycoses 2012, 55, 161–167. [Google Scholar] [CrossRef]
- Wang, X.; Cai, W.; Gerrits van den Ende, B.; Zhang, J.; Xie, T.; Xi, L.; Li, X.; Sun, J.; de Hoog, S. Indoor wet cells as a habitat for melanized fungi, opportunistic pathogens on humans and other vertebrates. Sci. Rep. 2018, 8, 7685. [Google Scholar] [CrossRef] [PubMed]






| Sample | Observed Richness | Chao1 Estimator | Shannon Index | Simpson Index |
|---|---|---|---|---|
| S1 | 11 | 14 | 1.23 | 0.36 |
| S2 | 12 | 14 | 0.67 | 0.66 |
| S3 | 3 | 3 | 0.02 | 0.99 |
| S4 | 6 | 6 | 1.29 | 0.30 |
| S5 | 19 | 19 | 1.07 | 0.45 |
| S6 | 15 | 15 | 1.67 | 0.25 |
| S7 | 6 | 6 | 0.47 | 0.78 |
| S8 | 8 | 8 | 0.72 | 0.64 |
| S9 | 10 | 10 | 0.95 | 0.55 |
| S10 | 6 | 7 | 0.50 | 0.71 |
| S11 | 20 | 20 | 0.58 | 0.77 |
| S12 | 4 | 4 | 0.90 | 0.51 |
| S13 | 9 | 10 | 0.46 | 0.81 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Precha, N.; Kliengchuay, W.; Woo, C.; Yamamoto, N.; Tantrakarnapa, K. Fungal Assemblages on Indoor Surfaces with Visible Mold Growth in Homes after the 2016 Flood Disaster in Thailand. Appl. Sci. 2020, 10, 5322. https://doi.org/10.3390/app10155322
Precha N, Kliengchuay W, Woo C, Yamamoto N, Tantrakarnapa K. Fungal Assemblages on Indoor Surfaces with Visible Mold Growth in Homes after the 2016 Flood Disaster in Thailand. Applied Sciences. 2020; 10(15):5322. https://doi.org/10.3390/app10155322
Chicago/Turabian StylePrecha, Nopadol, Wissanupong Kliengchuay, Cheolwoon Woo, Naomichi Yamamoto, and Kraichat Tantrakarnapa. 2020. "Fungal Assemblages on Indoor Surfaces with Visible Mold Growth in Homes after the 2016 Flood Disaster in Thailand" Applied Sciences 10, no. 15: 5322. https://doi.org/10.3390/app10155322