Abstract
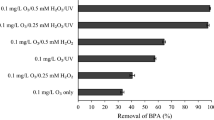
To estimate greenhouse gas (GHG) emissions and degradation rate constants (kobs) from H2O2/UV-C, TiO2/UV-C, and ozonation processes in the degradation of bisphenol A (BPA), the laboratory scale experiments were conducted. In the H2O2/UV-C process, the fastest degradation rate constant (kobs = 0.353 min−1) was observed at 4 mM of H2O2, while the minimum GHG emission was achieved at 3 mM of H2O2. In the TiO2/UV-C process, the fastest rate constant (kobs = 0.126 min−1) was achieved at 2000 mg/L of TiO2, while the minimum GHG emission was observed at 400 mg/L of TiO2. In the ozonation process, GHG emissions were minimal at 5 mg/L of O3, but the degradation rate constant kept on increasing as the O3 concentration increased. There were three major types of GHG emissions in the advanced oxidation processes (AOPs). In the ozonation process, most of the GHG emissions were generated by electricity consumption. TiO2/UV-C process accounted for a significant portion of the GHGs generated by the use of chemicals. Finally, the H2O2/UV-C process produced similar GHG emissions from both chemical inputs and electricity consumption. The carbon footprint calculation revealed that for the treatment of 1 m3 of water contaminated with 0.04 mM BPA, the H2O2/UV-C process had the smallest carbon footprint (0.565 kg CO2 eq/m3), followed by the TiO2/UV-C process (3.445 kg CO2 eq/m3) and the ozonation process (3.897 kg CO2 eq/m3). Our results imply that the increase in removal rate constant might not be the optimal parameter for reducing GHG emissions during the application of these processes.

.





Similar content being viewed by others
References
Beltran FJ (2003) Ozone reaction kinetics for water and wastewater systems. CRC Press
Biń AK, Sobera-Madej S (2012) Comparison of the advanced oxidation processes (UV, UV/H2O2, and O3) for the removal of antibiotic substances during wastewater treatment. Ozone Sci Eng 34(2):136–139
Bolton JR, Bircher KG, Tumas W, Tolman CA (2001) Figures-of-merit for the technical development and application of advanced oxidation technologies for both electric-and solar-driven systems (IUPAC Technical Report). Pure Appl Chem 73(4):627–637
Broséus R, Vincent S, Aboulfadl K, Daneshvar A, Sauvé S, Barbeau B, Prévost M (2009) Ozone oxidation of pharmaceuticals, endocrine disruptors and pesticides during drinking water treatment. Water Res 43(18):4707–4717
Drewes J, Khan S (2015) Contemporary design, operation, and monitoring of potable reuse systems. J Water Reuse Desalination 5(1):1–7
Eggleston S, Buendia L, Miwa K, Ngara T and Tanabe K (2006) 2006 IPCC guidelines for national greenhouse gas inventories, Institute for Global Environmental Strategies Hayama, Japan
El-Fadel M, Massoud M (2001) Methane emissions from wastewater management. Environ Pollut 114(2):177–185
Frischknecht R, Jungbluth N, Althaus H-J, Hischier R, Doka G, Dones R, Heck T, Hellweg S, Wernet G and Nemecek T (2007) Overview and methodology. Data v2. 0 (2007). Ecoinvent report No. 1, Ecoinvent centre
Garoma T, Matsumoto S (2009) Ozonation of aqueous solution containing bisphenol A: effect of operational parameters. J Hazard Mater 167(1–3):1185–1191
Griffith DR, Barnes RT, Raymond PA (2009) Inputs of fossil carbon from wastewater treatment plants to US rivers and oceans. Environ Sci Technol 43(15):5647–5651
Gu Y, Dong Y-n, Wang H, Keller A, Xu J, Chiramba T, Li F (2016) Quantification of the water, energy and carbon footprints of wastewater treatment plants in China considering a water–energy nexus perspective. Ecol Indic 60:402–409
Gultekin I, Mavrov V, Ince NH (2009) Degradation of bisphenol-A by ozonation. J Adv Oxid Technol 12(2):242–248
Hwang K-L, Bang C-H, Zoh K-D (2016) Characteristics of methane and nitrous oxide emissions from the wastewater treatment plant. Bioresour Technol 214:881–884
Im J-K, Son H-S, Kang Y-M, Zoh K-D (2012) Carbamazepine degradation by photolysis and titanium dioxide photocatalysis. Water Environ Res 84(7):554–561
Kanakaraju D, Glass BD, Oelgemöller M (2018) Advanced oxidation process-mediated removal of pharmaceuticals from water: a review. J Environ Manag 219:189–207
Kaneco S, Rahman MA, Suzuki T, Katsumata H, Ohta K (2004) Optimization of solar photocatalytic degradation conditions of bisphenol A in water using titanium dioxide. J Photochem Photobiol A Chem 163(3):419–424
Kang Y-M, Kim M-K, Zoh K-D (2018) Effect of nitrate, carbonate/bicarbonate, humic acid, and H2O2 on the kinetics and degradation mechanism of bisphenol-A during UV photolysis. Chemosphere 204:148–155
Kang Y-M, Kim M-K, Kim T, Kim T-K, Zoh K-D (2019) Occurrence and fate of micropollutants in private wastewater treatment facility (WTF) and their impact on receiving water. Environ Manag 64(5):650–660
KEITI (2003) Korea Environmental Industry & Technology Institute
Kim T-K, Kim T, Jo A, Park S, Choi K, Zoh K-D (2018) Degradation mechanism of cyanide in water using a UV-LED/H2O2/Cu2+ system. Chemosphere 208:441–449
Mander Ü, Dotro G, Ebie Y, Towprayoon S, Chiemchaisri C, Nogueira SF, Jamsranjav B, Kasak K, Truu J, Tournebize J (2014) Greenhouse gas emission in constructed wetlands for wastewater treatment: a review. Ecol Eng 66:19–35
Mannina G, Ekama G, Caniani D, Cosenza A, Esposito G, Gori R, Garrido-Baserba M, Rosso D, Olsson G (2016) Greenhouse gases from wastewater treatment—a review of modelling tools. Sci Total Environ 551:254–270
Masuda S, Sano I, Hojo T, Li Y-Y, Nishimura O (2018) The comparison of greenhouse gas emissions in sewage treatment plants with different treatment processes. Chemosphere 193:581–590
Meneses M, Pasqualino JC, Castells F (2010) Environmental assessment of urban wastewater reuse: treatment alternatives and applications. Chemosphere 81(2):266–272
Miklos DB, Remy C, Jekel M, Linden KG, Drewes JE, Hübner U (2018) Evaluation of advanced oxidation processes for water and wastewater treatment–aa critical review. Water Res 139:118–131
Mohapatra D, Brar S, Tyagi R, Surampalli R (2010) Physico-chemical pre-treatment and biotransformation of wastewater and wastewater sludge–fate of bisphenol A. Chemosphere 78(8):923–941
Munoz I, Rodriguez A, Rosal R, Fernandez-Alba AR (2009) Life cycle assessment of urban wastewater reuse with ozonation as tertiary treatment: a focus on toxicity-related impacts. Sci Total Environ 407(4):1245–1256
Muruganandham M, Swaminathan M (2004) Photochemical oxidation of reactive azo dye with UV-H2O2 process. Dyes Pigments 62(3):269–275
Park C, Choi E, Jeon H, Lee J, Sung B, Cho Y, Ko K (2014) Effect of nitrate on the degradation of bisphenol A by UV/H2O2 and ozone/H2O2 oxidation in aqueous solution. Desalin Water Treat 52(4–6):797–804
Parravicini V, Svardal K, Krampe J (2016) Greenhouse gas emissions from wastewater treatment plants. Energy Procedia 97:246–253
Pasqualino JC, Meneses M, Castells F (2011) Life cycle assessment of urban wastewater reclamation and reuse alternatives. J Ind Ecol 15(1):49–63
Raluy R, Serra L, Uche J (2005) Life cycle assessment of desalination technologies integrated with renewable energies. Desalination 183(1–3):81–93
Reddy PVL, Kim K-H, Kavitha B, Kumar V, Raza N, Kalagara S (2018) Photocatalytic degradation of bisphenol A in aqueous media: a review. J Environ Manag 213:189–205
Riga A, Soutsas K, Ntampegliotis K, Karayannis V, Papapolymerou G (2007) Effect of system parameters and of inorganic salts on the decolorization and degradation of Procion H-exl dyes. Comparison of H2O2/UV, Fenton, UV/Fenton, TiO2/UV and TiO2/UV/H2O2 processes. Desalination 211(1–3):72–86
Robescu LD, Presură E (2017) Reducing carbon footprint of a wastewater treatment plant using advanced treatment and renewable energy sources. Environ Eng Manag J (EEMJ) 16(5):1055–1062
Shahabadi MB, Yerushalmi L, Haghighat F (2009) Impact of process design on greenhouse gas (GHG) generation by wastewater treatment plants. Water Res 43(10):2679–2687
Sharma J, Mishra I, Kumar V (2015) Degradation and mineralization of Bisphenol A (BPA) in aqueous solution using advanced oxidation processes: UV/H2O2 and UV/S2O82− oxidation systems. J Environ Manag 156:266–275
Singh P, Kansal A, Carliell-Marquet C (2016) Energy and carbon footprints of sewage treatment methods. J Environ Manag 165:22–30
Tran BC, Teil MJ, Blanchard M, Alliot F, Chevreuil M (2015) BPA and phthalate fate in a sewage network and an elementary river of France. Influence of hydroclimatic conditions. Chemosphere 119:43–51
Tsai W-T, Lee M-K, Su T-Y, Chang Y-M (2009) Photodegradation of bisphenol-A in a batch TiO2 suspension reactor. J Hazard Mater 168(1):269–275
Umar M, Roddick F, Fan L, Aziz HA (2013) Application of ozone for the removal of bisphenol A from water and wastewater–a review. Chemosphere 90(8):2197–2207
Wang R, Ren D, Xia S, Zhang Y, Zhao J (2009) Photocatalytic degradation of bisphenol A (BPA) using immobilized TiO2 and UV illumination in a horizontal circulating bed photocatalytic reactor (HCBPR). J Hazard Mater 169(1–3):926–932
Wong C, Chu W (2003) The direct photolysis and photocatalytic degradation of alachlor at different TiO2 and UV sources. Chemosphere 50(8):981–987
Funding
This study was supported by the Korea Environmental Industry & Technology Institute (KEITI) through the project for developing innovative drinking water and wastewater technologies funded by Korea Ministry of Environment (MOE) (NO. 2019002710001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Vítor Pais Vilar
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• We estimated GHG emission during H2O2/UV, TiO2/UV, and O3 reactions of bisphenol A.
• Carbon footprint concept was used to compare GHG emission in different AOP processes.
• Conditions to increase the rate constant may not be optimal in terms of GHG emission.
• H2O2/UV process showed the smallest carbon footprint among the AOP processes.
Rights and permissions
About this article
Cite this article
Kang, YM., Kim, TK., Kim, MK. et al. Greenhouse gas emissions from advanced oxidation processes in the degradation of bisphenol A: a comparative study of the H2O2/UV, TiO2 /UV, and ozonation processes. Environ Sci Pollut Res 27, 12227–12236 (2020). https://doi.org/10.1007/s11356-020-07807-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-07807-3